Question 1: Describe the spectrum of hydrogen in detail
ANSWER
Spectrum of H atom
Hydrogen atom is said to be stable when its electron revolves in the first orbit of principle quantum number, n = 1. When this electron gains energy from the surrounding, it jumps into a higher orbit with principle quantum number n = 1, 2, 3… . The atom is said to be excited and the higher orbit is unstable and the electron jumps back from the higher to the lower orbit; emitting the energy of value hf.
It should be noted that, although Hydrogen atom consists of a single electron, still it has a large number of outer shells. When the electron absorbs energy it jumps to any of the high energy orbits. When the energized unstable electron jumps back to a lower orbit, it emits energy as electromagnetic radiation. The associated wavelength λ of the photon depends upon the energy difference of the orbits in which the transition takes place. Since there are many empty shells available for the transition, we observe multiple line spectrum of the mono-atomic Hydrogen atom.
Experiment
Consider a discharge tube containing hydrogen gas. Let a high voltage is applied across the tube. The gas becomes luminous, giving off a bluish-red light. When this light from the tube is passed through a prism, it can be analyzed by the spectrum of the colors produced. Each line of the spectrum represents a wavelength emitted by the source.
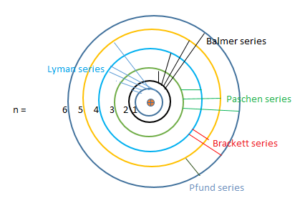
So the line spectrum of Hydrogen consists series of lines in the infrared, visible and ultraviolet regions. In 1885, John Balmer found the wavelengths of these lines could be described by the equation,
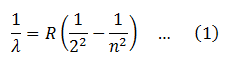
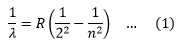
Where R is constant, called Rydberg constant. If the wavelength is in meters its value is 1.0973732*107/m.
The general form of the equation covering all series is,
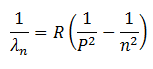
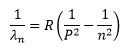
Where P and n are the initial (higher) and final (lower) energy states of the transition. A description of the series of the spectrum is as follow.
(1) Lyman series: This series is in the ultraviolet region and the transition takes place in the first orbit from the outer orbits.
(2) Balmer series: This series is in the visible region and the transition takes place in the 2nd orbit from the outer orbits.
(3) Paschen series: It lies in the infrared region and the transition takes place between the 3rd and outer orbits.
(4) Brackett series: It lies in the infrared region and the transition takes place in the 4th orbit from the outer orbits.
(5) Pfund series: This series is in the far infrared region and the transition takes place fall in the 5th orbit from the outer orbits.


Pingback:bohr-postulates-about-h-atom – msa
Pingback:mcqs-atomic-spectra-p-12 – msa
Pingback:long-questions-ch19-p12