Question 2: Explain the principle, construction, working and necessary mathematical theory of mass spectrometer.
ANSWER
Mass spectrometer
Mass spectrometer is a device with the help of which we separate different isotopes of an element and measure their masses.
Principle
A mass spectrometer is making use of an electric and magnetic field. The electric field accelerates the ions and the magnetic field deflects them according their masses. Masses of various isotopes are determined by the extent they are deflected in the magnetic field.
Construction
It consists of the following parts.
- Source of ions: Atoms or molecules of the element under investigation are ionized in an ion source, S. Positive ions then enter the electric field
- Electric Field: An electric field is provided between two slits S1 and S2. Ions are accelerated in this region.
- Magnetic field and vacuum chamber: Ions are deflected in the magnetic field according to their masses as they enter the magnetic field with some velocity gained in the electric field.
- Photographic Plate: The deflected ions hit the photographic plate and their position is detected
Working and necessary mathematics:
Positive ions from the ion source are allowed to enter the electric field through slit S1, where they are accelerated towards slit S2. If V is the potential difference and q is the charge on the particle (ion), then
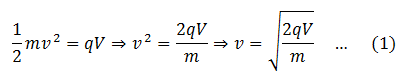
The ions pass through slit S2 with this velocity and enter a vacuum chamber. A magnetic field is normally provided in this area which deflects the ions. The magnetic force experienced by the ion having charge q is Fm = qvB, where B is the strength of the magnetic field. The ion is deflected under the action of this force and it is equal to the centripetal force on the ion to make it move in the circular path. Hence,
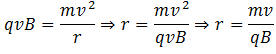
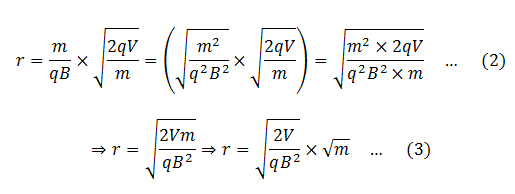
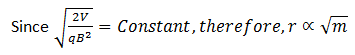
We know that different isotopes of an element have different masses. So if the ions in the experiment have different masses (or the element under investigation has different isotopes), they will deflect differently. Ions with higher masses would have larger radii and ions with smaller masses would smaller radii. Equivalently, ions with lighter masses would deflect more than the ions with larger masses.
Value of r (which gives the extent of deflection) is determined by measuring the distance between slit S2 and the image produced on the photographic plate. As ions deflected with different angles will hit on different places, therefore, different values of r are determined.
From equations (3)
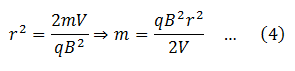
Since q, r, B and V are all known, masses of different isotopes present in the element under investigation can be determined by putting the respective value of r.

Pingback:isotopes – msa
Pingback:natural-radioactivity-and-its-types – msa
Pingback:long-questions-ch20-p12 – msa