Question 6: What is de’ Broglie hypothesis? Describe an experiment to show that particle has wave characteristics.
ANSWER
De Broglie Hypothesis:
De Broglie, in 1924, gave the hypothesis that if light can show to have matter (or particle) properties then matter must have wave properties. He argued that nature reveals symmetries so the wave nature of matter must be in association with the particle nature of waves.
He then worked to find a relation for the wavelength associated with matter. He argued that energy of a photon of frequency f is
E = hf … (1)
Einstein mass energy relation is
E = mc2 … (2)
Therefore, from (1) and (2)
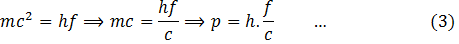
This equation gives the momentum of photon in terms of frequency. Similarly,
c = fλ OR λ = c/f OR 1/λ = f/c … (4)
Put this value in (3)
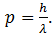
This equation gives the momentum of photon in terms of wavelength. De’ Broglie argued that in the same way, the wavelength λ assigned to a particle having mass m and velocity v is
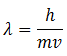
However, for larger particles the wavelength is so small that it cannot be detected. Whereas for small subatomic particles the wavelength can be determined.
Example
Take a cricket ball of mass 0.25 kg. When hit with the bat, let it goes off with a velocity of 20 m/s. Then

This is too small to be detected.
Now take the case of an electron moving with a speed of 106 m/s. Then
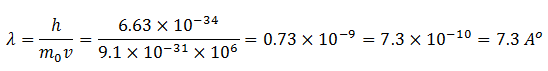
This wavelength is in the order of the wavelength of X-rays and detectable.
Davisson and Germer Experiment
Wave nature of particle was established by Davission and Germer.
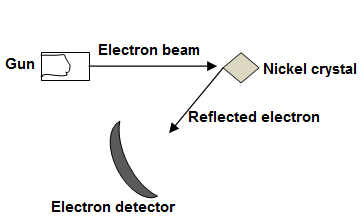
The apparatus they used was arranged in a vacuum chamber as shown in the diagram. Electrons from an electron gun were incident on a single Nickel crystal. After falling on the crystal, the electrons were diffracted by the crystal and were detected by a movable electron detector.
Since diffraction is wave property, therefore, this proved electron possesses wave properties as well.

Pingback:wave-particle-duality – msa
Pingback:pair-production-and-annihilation-of-matter – msa
Pingback:long-questions-ch-18-p12 – msa